O2 is the scientific formula for carbon dioxide and occurs due to a reaction between the elements carbon (C) and oxygen (O) . Carbon and oxygen are 2 of the most important building stones of life for any organism.
What is the relationship between tanks and CO2? To keep it simple it’s something like this :
CO2 is used and broken down by aquatic plants. The carbon is used as an ingredient for their own growth. The oxygen will be returned to the water column which is important for all live organisms that depend on the presence of oxygen in the water. This event happens under the influence of light, what we call photosynthesis. When the lights are out the process stops and plants will use oxygen and release carbon dioxide as well.
CO2 is a building stone for the biomass (bacteria) in a biological filter as well.
To see to it that we have an optimum amount of carbon dioxide in the water column we often use CO2 fertilisation to our benefit. A side effect of CO2 fertilisation is that the pH will lower mildly because CO2 reacts with water creating a weak acid named H2CO3 better known as carbonic acid.
But as with everything :
To much of the good stuff (CO2 is very dissolvable in water) can have some not wanted side effects and even can become poisonous. So being alert is very important !!
What if we don’t use CO2 fertilisation and there is not much dissolved in the water column ??
Plants are going to use the available carbonates and bi carbonate ions to have access to the much needed carbon .
Carbonate- and bi carbonate ions are available in the water and substrate (some more, some less).
The counter point of all this is that it decreases the carbonate hardness (KH) in the water column.
The carbonate hardness, on its own, is the buffering capacity of water.
This could mean that when the KH decreases in the aquarium’s water, the pH gets unstable (fluctuates) with all the side effects with it .
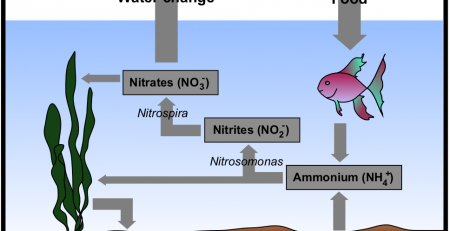
It is a matter of importance to measure the amount of CO2 regularly and keep it on a reasonable level. This can be done with CO2 fertilisation. Other producers of CO2 are the fish and the plants during the night. Even a biological filter releases CO2 into the water. With the break down of the produced waste matter within the filtration system and tank, a lot of carbon dioxide is formed.
That is why a properly functioning bio filtration is a must for all tank keepers.
How much CO2 is the appropriate amount for a planted tank ?
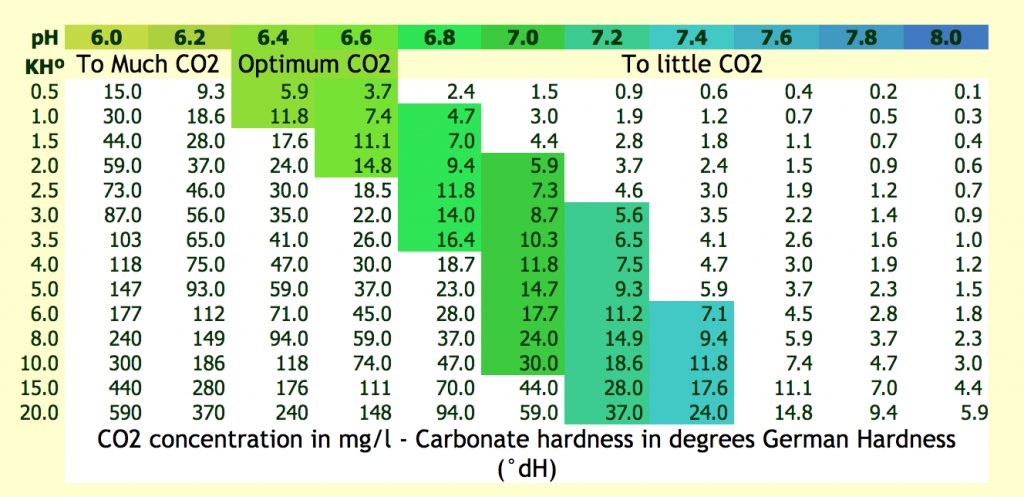
the table below gives us a clear insight.
CO2 concentration in mg/l – Carbonate hardness in degrees German Hardness (°dH)
Now you could say that these figures are constants, but to be honest, they aren’t.
These figures are under the constant influence of, as we said before, salts but light and acids as well.
Picture f.i. a normal planted tank with exactly the right amount of fish. The KH parameter is exactly 4.0 dH without CO2 addition. Because there is no CO2 addition the plants will use up all CO2 in a lights on situation and the pH will rise. The salts (carbonates and bi carbonates ) will keep this all under control within a certain level (buffering capacity).
In the table above you can see that when the KH is 4 dH and the pH is 7.0 the amount of free CO2 dissolved in water is 11.8 mg/l. That certain pH is under influence of carbon addition. By this time I hear a lot of people saying: “what are you moaning about” ?? This is an ideal situation ! Well I could say: just forget it !!
For “normal” Dutch tanks maybe ! The fish and plants of these certain tanks are more used to fluctuations due their native habitat where fluctuations are “normal“ during the time of year.
But we have to avoid these fluctuations in our discus tanks. The parameters in the Amazon doesn’t change that much only a slight change during the rainy season.
For a lot of aquarists its normal to connect the CO2 equipment and let it run day and night.
This is so wrong !! During the night there is no CO2 usage but plants, fish and filter create more CO2 and therefore the pH can change dramatically.
So how do we leverage CO2?
The easiest way is to buy the complete equipment with all these needed gadgets.
To be more specific:
The standard CO2 equipment including a pH monitor and magnet valve for the proper CO2 dosage.
This way nothing really changes.
The pH is constantly checked and adjusted when it’s needed.
Sadly this is the most expensive way as well .
But then again we spent an awful amount of money on our beloved treasures but are saving on it’s house and garden .
Never forget that once you started with discus in a planted tank it’s supposed to be a long term relationship.
Use the upper table to decide if you really need CO2 addition. When you found out that the amount of CO2 is between the 10 mg/l and 30 mg/l nothing can’t go wrong.
Never use CO2 as an easy way to acidify your water rigorously, this will definitely go wrong .
See it more as an addition of your fertilisation regime with, as a beneficial side effect, mild lowering of the pH.
Now the definite: at last!!
The lower the KH, the more benefit you have from your CO2 addition with one restriction!!
Keep it safe ! Try to maintain the KH level above 4 dH.









Leave a Reply
You must be logged in to post a comment.